Welcome to the comprehensive stoichiometry test review answer key, your ultimate guide to mastering chemical calculations. Dive into the world of balanced equations, mole conversions, limiting reactants, and more, empowering you to conquer any stoichiometry challenge with confidence.
Throughout this review, we’ll explore the intricacies of stoichiometry, providing step-by-step guidance and practice problems to enhance your understanding. Get ready to unlock the secrets of chemical reactions and excel in your stoichiometry endeavors.
1. Definition and Overview of Stoichiometry
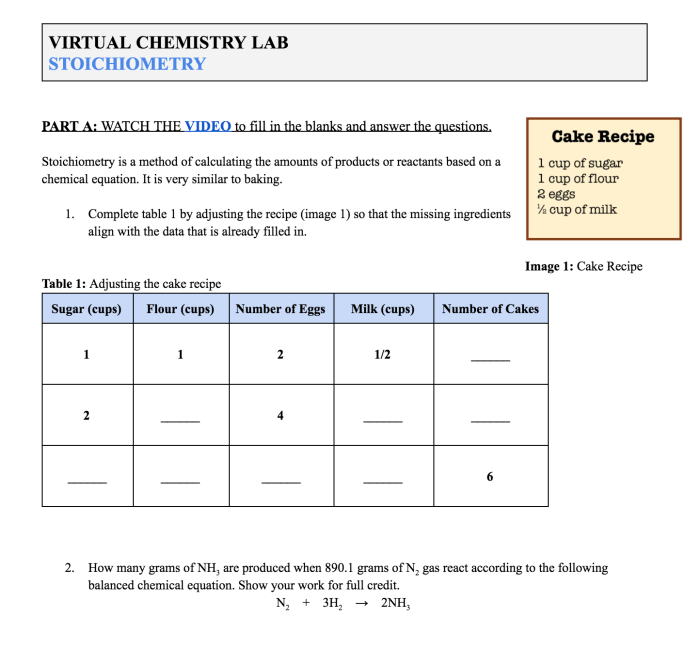
Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It provides a framework for understanding and predicting the amounts of substances involved in a chemical change. Stoichiometry is essential for various applications in chemistry, including industrial processes, environmental monitoring, and medical research.
Examples of Stoichiometric Calculations in Real-World Scenarios
- Calculating the amount of fuel required to power a car
- Determining the optimal fertilizer composition for crop growth
- Predicting the amount of carbon dioxide produced by a combustion reaction
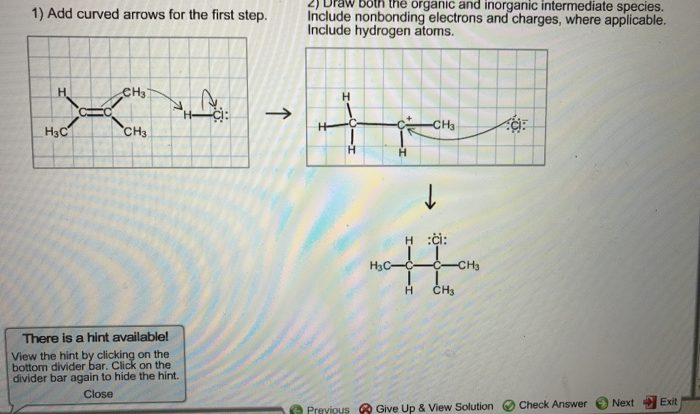
2. Balancing Chemical Equations
Steps Involved in Balancing Chemical Equations
- Write the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the coefficients in front of each compound to make the number of atoms of each element equal on both sides.
Importance of Coefficients in Stoichiometric Calculations
The coefficients in a balanced chemical equation represent the mole ratios of the reactants and products. These ratios are crucial for determining the quantitative relationships between the substances involved in the reaction.
3. Mole Calculations
Concept of a Mole and Avogadro’s Number
A mole is the SI unit of amount of substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12. Avogadro’s number, represented by N A, is the number of elementary entities (atoms, molecules, ions, or electrons) in one mole of a substance and is equal to 6.022 × 10 23.
Converting Between Grams, Moles, and Molecules
- Grams to moles:Divide the mass by the molar mass.
- Moles to grams:Multiply the number of moles by the molar mass.
- Moles to molecules:Multiply the number of moles by Avogadro’s number.
4. Limiting Reactant and Excess Reactant
Concept of Limiting Reactant and Excess Reactant
In a chemical reaction, the limiting reactant is the reactant that is completely consumed, while the excess reactant is the reactant that remains after the reaction is complete. The limiting reactant determines the maximum amount of product that can be formed.
Identifying the Limiting Reactant
To identify the limiting reactant, compare the mole ratios of the reactants to their stoichiometric coefficients. The reactant with the smallest mole ratio is the limiting reactant.
5. Stoichiometry in Gas Reactions: Stoichiometry Test Review Answer Key
Relationship Between Moles and Volume in Gas Reactions
At constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of gas present. This relationship is known as Avogadro’s law.
Using Stoichiometry to Calculate the Volume of Gases Produced or Consumed
The stoichiometric coefficients in a balanced chemical equation can be used to determine the volume of gases produced or consumed in a reaction.
6. Stoichiometry in Solution Reactions
Concept of Molarity and Solution Concentration
Molarity is a measure of the concentration of a solution. It is defined as the number of moles of solute per liter of solution. The SI unit of molarity is mol/L.
Using Stoichiometry to Calculate the Concentration of Solutions, Stoichiometry test review answer key
The stoichiometric coefficients in a balanced chemical equation can be used to calculate the concentration of a solution if the amount of solute and the volume of the solution are known.
7. Percent Yield and Error Analysis
Definition of Percent Yield
Percent yield is a measure of the efficiency of a chemical reaction. It is defined as the ratio of the actual yield to the theoretical yield, multiplied by 100.
Common Sources of Error in Stoichiometry Calculations
- Measurement errors
- Errors in balancing chemical equations
- Errors in mole conversions
8. Applications of Stoichiometry
Practical Applications of Stoichiometry in Various Fields
- Industrial chemistry:Designing and optimizing chemical processes
- Environmental chemistry:Monitoring and controlling pollution
- Medicine:Calculating drug dosages and designing drug delivery systems
- Food science:Developing new food products and optimizing food processing techniques
FAQ Guide
What is the significance of stoichiometry in chemistry?
Stoichiometry is crucial in chemistry as it allows us to predict the quantitative relationships between reactants and products in chemical reactions. It helps us determine the exact amounts of reactants needed and products formed, ensuring efficient and accurate chemical processes.
How do I balance chemical equations?
Balancing chemical equations involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation. This reflects the law of conservation of mass and ensures that the reaction is chemically feasible.
What is the concept of a limiting reactant?
In a chemical reaction, the limiting reactant is the reactant that is entirely consumed, determining the maximum amount of product that can be formed. Identifying the limiting reactant is essential for predicting the reaction’s outcome and optimizing resource utilization.