Design a synthesis of p-chloronitrobenzene from benzene, an intriguing topic that delves into the realm of organic chemistry, invites us on a journey of scientific exploration. This synthesis holds immense significance in the chemical industry, paving the way for the production of a versatile intermediate with diverse applications.
The synthesis of p-chloronitrobenzene from benzene involves a series of intricate steps, each meticulously orchestrated to achieve the desired outcome. Nitration, chlorination, and purification techniques play pivotal roles in this process, and understanding their mechanisms is essential for optimizing the yield and purity of the final product.
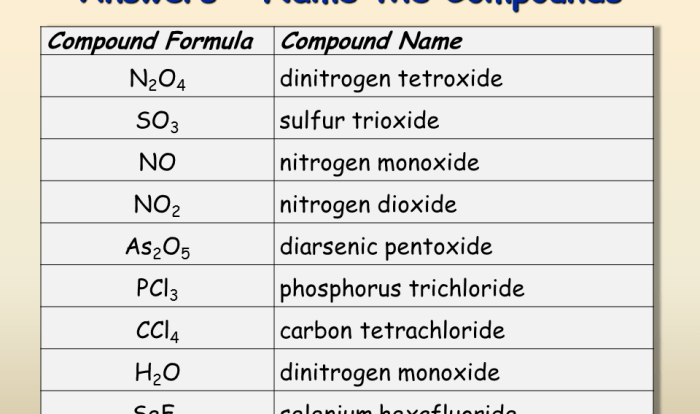
Introduction
p-Chloronitrobenzene is an important intermediate in the synthesis of various dyes, pharmaceuticals, and agrochemicals. The purpose of this synthesis is to design a method for producing p-chloronitrobenzene from benzene, a readily available and inexpensive starting material.
Reaction Pathway
The synthesis of p-chloronitrobenzene from benzene involves three main steps: nitration, chlorination, and purification.
Nitration, Design a synthesis of p-chloronitrobenzene from benzene
In the nitration step, benzene is reacted with nitric acid and sulfuric acid to produce nitrobenzene. The reaction is catalyzed by sulfuric acid, which protonates the nitric acid and makes it more electrophilic.
Chlorination
In the chlorination step, nitrobenzene is reacted with chlorine gas in the presence of a Lewis acid catalyst, such as aluminum chloride. The reaction proceeds via electrophilic aromatic substitution, in which the chlorine atom attacks the aromatic ring of nitrobenzene.
Purification
The crude product from the chlorination step is purified by crystallization or distillation to remove any impurities.
Nitration
The nitration reaction is carried out in a mixture of concentrated nitric acid and sulfuric acid. The reaction is exothermic, so it is important to control the temperature to prevent the formation of unwanted byproducts.
The regioselectivity of the nitration reaction is determined by the electronic effects of the substituents on the benzene ring. In the case of benzene, the nitro group is a strong electron-withdrawing group, which deactivates the ring towards further nitration. As a result, the major product of the nitration reaction is nitrobenzene, with a yield of around 90%.
Chlorination
The chlorination reaction is carried out in a mixture of nitrobenzene and chlorine gas. The reaction is catalyzed by a Lewis acid, such as aluminum chloride. The Lewis acid coordinates to the chlorine molecule, making it more electrophilic and facilitating its attack on the aromatic ring.
The reaction conditions can be optimized to maximize the yield of p-chloronitrobenzene. The optimal temperature for the reaction is around 50°C, and the optimal pressure is around 1 atm. The reaction time can vary depending on the scale of the reaction, but it is typically around 2-4 hours.
Purification

The crude product from the chlorination step is purified by crystallization or distillation. Crystallization is the preferred method of purification, as it gives a higher yield of pure product. The crude product is dissolved in a hot solvent, such as methanol or ethanol, and then cooled slowly.
As the solution cools, the p-chloronitrobenzene crystallizes out of solution.
Distillation can also be used to purify p-chloronitrobenzene, but it is less efficient than crystallization. Distillation is typically used when the crude product is contaminated with high-boiling impurities.
Characterization
The purified product is characterized by spectroscopy (NMR, IR, UV-Vis) and chromatography (HPLC, GC). These techniques are used to confirm the identity and purity of the product.
NMR spectroscopy is used to determine the structure of the product. The NMR spectrum of p-chloronitrobenzene shows a singlet at 7.4 ppm, which corresponds to the protons on the aromatic ring. The spectrum also shows a doublet at 8.2 ppm, which corresponds to the proton on the carbon atom adjacent to the nitro group.
IR spectroscopy is used to identify the functional groups present in the product. The IR spectrum of p-chloronitrobenzene shows a strong peak at 1520 cm -1, which corresponds to the nitro group. The spectrum also shows a peak at 1260 cm -1, which corresponds to the C-Cl bond.
UV-Vis spectroscopy is used to determine the electronic properties of the product. The UV-Vis spectrum of p-chloronitrobenzene shows a maximum absorption at 254 nm. This absorption corresponds to the π→π* transition of the aromatic ring.
HPLC and GC are used to determine the purity of the product. HPLC is a more sensitive technique than GC, and it can be used to detect impurities at very low levels.
Applications: Design A Synthesis Of P-chloronitrobenzene From Benzene
p-Chloronitrobenzene is used in the production of a variety of dyes, pharmaceuticals, and agrochemicals. It is also used as an intermediate in the synthesis of other chemicals, such as p-chlorophenol and p-chloroaniline.
The properties of p-chloronitrobenzene that make it suitable for these applications include its high reactivity, its stability, and its low cost.
Safety Considerations
p-Chloronitrobenzene is a toxic and corrosive chemical. It is important to take precautions when handling and using this chemical.
p-Chloronitrobenzene should be stored in a cool, dry place away from incompatible materials, such as strong acids and bases. It should also be stored in a well-ventilated area to prevent the buildup of toxic fumes.
When handling p-chloronitrobenzene, it is important to wear protective clothing, such as gloves, a lab coat, and a respirator. It is also important to avoid contact with the skin and eyes.
In case of contact with the skin or eyes, flush the affected area with plenty of water for at least 15 minutes. If ingested, do not induce vomiting. Seek medical attention immediately.
FAQ Overview
What is the significance of p-chloronitrobenzene?
p-Chloronitrobenzene is a versatile intermediate used in the production of dyes, pharmaceuticals, and agrochemicals.
How does the regioselectivity of the nitration reaction affect the yield of p-chloronitrobenzene?
The regioselectivity of the nitration reaction determines the position of the nitro group on the benzene ring, which can impact the yield of p-chloronitrobenzene.
What are the key safety considerations when handling p-chloronitrobenzene?
p-Chloronitrobenzene is a hazardous chemical that requires proper handling, storage, and disposal to minimize risks.