Draw both the organic and inorganic intermediate species – In the realm of chemistry, intermediate species play a pivotal role in facilitating chemical reactions. This guide delves into the fascinating world of organic and inorganic intermediate species, exploring their characteristics, significance, and techniques for drawing them.
Organic intermediate species, characterized by their carbon-based structures, are crucial in organic synthesis. Inorganic intermediate species, on the other hand, lack carbon atoms and play a vital role in inorganic reactions.
Intermediate Species in Chemical Reactions
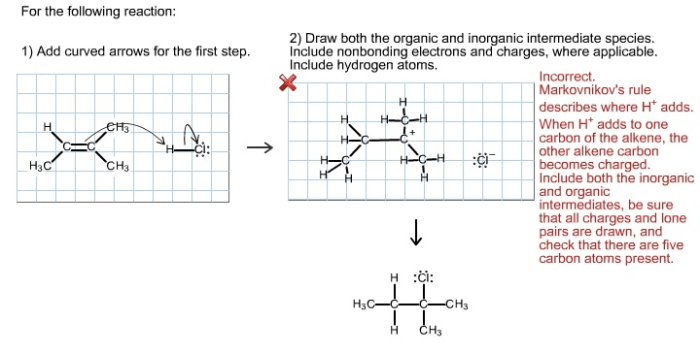
Intermediate species are short-lived, unstable chemical species that are formed during the course of a chemical reaction. They are not present in the reactants or products of the reaction but play a crucial role in the reaction mechanism.
Organic Intermediate Species
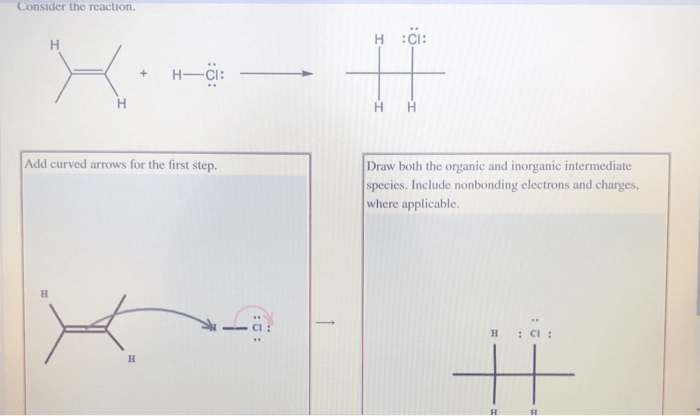
Organic intermediate species are molecules that contain carbon and are formed during organic reactions. They are typically unstable and highly reactive, and they can undergo a variety of reactions to form the final products of the reaction.Some examples of organic intermediate species include:
- Carbocations
- Carbanions
- Free radicals
- Enols
- Imines
Organic intermediate species play an important role in organic reactions because they allow for the formation of complex molecules from simpler starting materials. They also provide a way for reactions to proceed through multiple steps, which can increase the selectivity and efficiency of the reaction.
Inorganic Intermediate Species
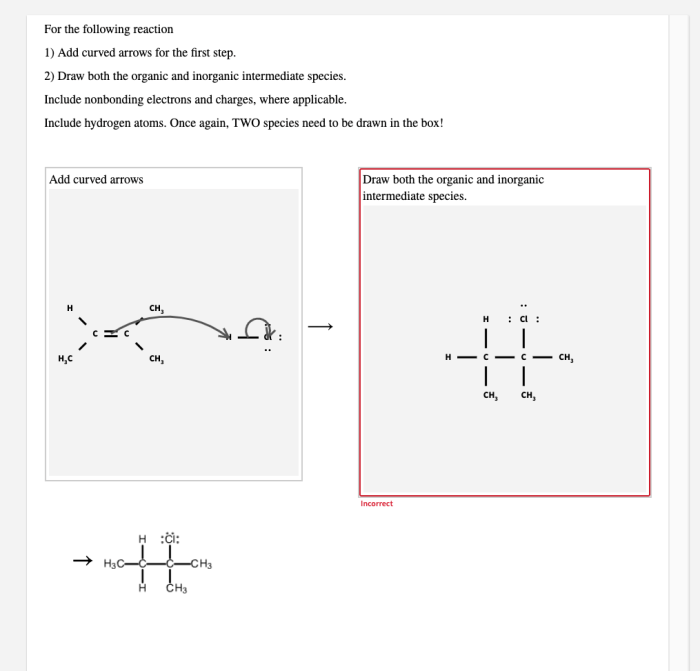
Inorganic intermediate species are molecules that do not contain carbon and are formed during inorganic reactions. They are typically less reactive than organic intermediate species, but they can still play an important role in the reaction mechanism.Some examples of inorganic intermediate species include:
- Metal complexes
- Oxides
- Hydroxides
- Acids
- Bases
Inorganic intermediate species play an important role in inorganic reactions because they allow for the formation of complex molecules from simpler starting materials. They also provide a way for reactions to proceed through multiple steps, which can increase the selectivity and efficiency of the reaction.
Drawing Organic and Inorganic Intermediate Species
Organic and inorganic intermediate species can be drawn using a variety of methods. The most common method is to use Lewis structures. Lewis structures show the arrangement of atoms and electrons in a molecule, and they can be used to represent both organic and inorganic intermediate species.When
drawing organic intermediate species, it is important to remember that they are typically unstable and highly reactive. This means that they will often have unpaired electrons, which can be represented by dots. It is also important to remember that organic intermediate species can undergo a variety of reactions, so it is important to draw them in a way that reflects their reactivity.When
drawing inorganic intermediate species, it is important to remember that they are typically less reactive than organic intermediate species. This means that they will often have all of their electrons paired. It is also important to remember that inorganic intermediate species can form a variety of complexes with other molecules, so it is important to draw them in a way that reflects their coordination chemistry.
Applications of Intermediate Species
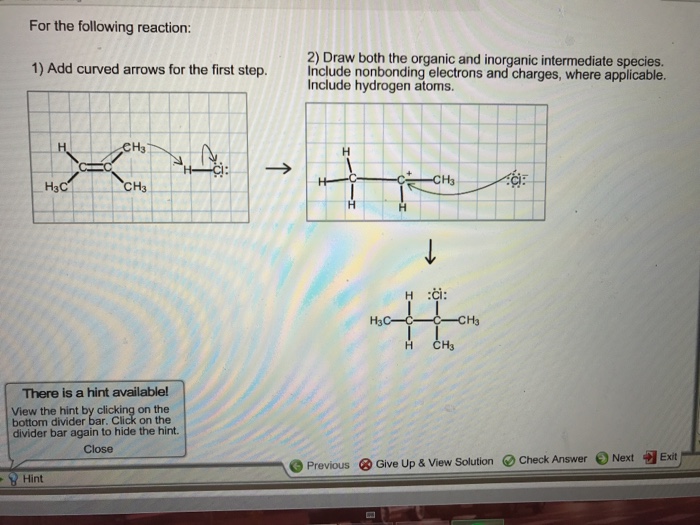
Intermediate species are used in a variety of applications, including:
Organic synthesis
Intermediate species are used in organic synthesis to create complex molecules from simpler starting materials.
Industrial chemistry
Intermediate species are used in industrial chemistry to produce a variety of products, including plastics, pharmaceuticals, and fuels.
Environmental chemistry
Intermediate species are used in environmental chemistry to study the fate of pollutants in the environment.
Helpful Answers: Draw Both The Organic And Inorganic Intermediate Species
What are the key differences between organic and inorganic intermediate species?
Organic intermediate species contain carbon atoms, while inorganic intermediate species do not.
Why is it important to draw intermediate species?
Drawing intermediate species helps visualize and understand the steps involved in chemical reactions.